I’m pleased to welcome back cardiologist Dr. Steven Nissen, recognized as one of Time Magazine’s World’s Most Influential People, for an update on clinical trials underway for treatment of elevated Lp(a) levels.
If you missed it, you can read the first installment of the interview, “Are you one of 1.4 billion people globally who have this dangerous disorder, but don’t know it?” at this link.
Lp(a) is a genetic disorder that impacts the masses.
This disorder, quietly lurking in many people’s bodies, is seldom discussed, nor understood, yet can play a significant role in future health.
“Lipoprotein(a), or Lp(a), is a distinctive particle with two components: a lipoprotein core that resembles LDL, along with a shell that contains apolipoprotein(a), or apo(a).”*
It is an easy diagnostic test that a doctor can order in your next blood draw. If you don’t know what it is, or what your level is, “that must change,” said Dr. Steven Nissen.
“Patients with Lp(a) concentrations greater than 150 nmo/L comprise approximately 20% of the general population and are associated with greatly increased risk of developing atherosclerotic cardiovascular disease and aortic valve stenosis.”**
The risk factors of Lp(a) are significant.
“Lp(a) has long been recognized as a genetically determined, independent risk factor for atherothrombotic cardiovascular disease.”**
Lp(a) risks can include*:
- Stroke
- Cardiovascular death
- Myocardial infarction
- Peripheral arterial disease
- Higher rate of aortic valve replacement
- More rapid progression of aortic stenosis
Studies and clinical trials have been underway in an effort to develop successful treatments to better the health and lessen cardiovascular events in individuals. There are currently no treatments approved by regulatory authorities.
Upon knowing your Lp(a) level, below is a chart for reference.


As Dr. Nissen stated in our first interview, “You could eat the best diet in the world and if you have high Lp(a), your blood levels don’t change.” It is genetically determined.
To put this in perspective, I am religious when it comes to eating healthy foods, mindfully exercising, and living a balanced lifestyle – yet I fall within that 20% of the population.
It is important to know what is in the pipeline and how we as individuals can lean in to live longer, more stable lives.
“In the absence of an effective Lp(a)-lowering drug therapy, this risk factor has traditionally been considered essentially untreatable.”**
It goes with saying why research efforts to find treatment options is crucial.
Nissen touched on three clinical trials underway. A brief summary follows.
1 – “The large ongoing phase III trial known as Lp(a) HORIZON announced by Novartis is now fully enrolled. We have 8,300 patients in 1,000 sites in 40 countries.” Half of the patients got the placebo and half got the drug.
Phase III in clinical trials “are what we call event-driven” trials, Nissen said. “Meaning that they run until you collect sufficient number of events to answer the scientific question. So, there is not a fixed timeframe.”
He continued, “The faster you accumulate events, the quicker the trial ends.”
HORIZON’s phase II trial showed its drug “reduced Lp(a) by up to 80% with weekly injections”… and “subsequent development as a monthly therapy.”**
2 – Amgen’s “short interfering RNAs (a different approach to lowering Lp(a) with a longer duration of action) is proceeding into phase III.” Further details have yet to be announced.
3 – “The Silence Therapeutics study (the “Study”) reported on [in this interview] is entering phase II soon.”
“We have a lot of shots in goal. We hope one of them ends up on the back of the net,” Nissen said. He stated that the clinical trials will likely go on for a number of years.
The Study included 32 participants with no known atherosclerotic cardiovascular disease and varying in age, body mass index, and Lp(a) levels.
Its approach “examined an alternative strategy to lowering Lp(a) using SLN360, a short interfering RNA (siRNA) to target LPA messenger RNA.”* “This approach results in selective uptake and concentration of SLN360 in hepatocytes, enabling the drug to bind and degrade the messenger RNA that encodes for apo(a).”**
The outcome: “SLN360, an siRNA targeting apo(a) production, was well tolerated and showed dose-dependent lowering of plasma Lp(a) concentrations.”**
The chart** below shows Lp(a) concentrations during the Study spanning from Baseline to Day 150.
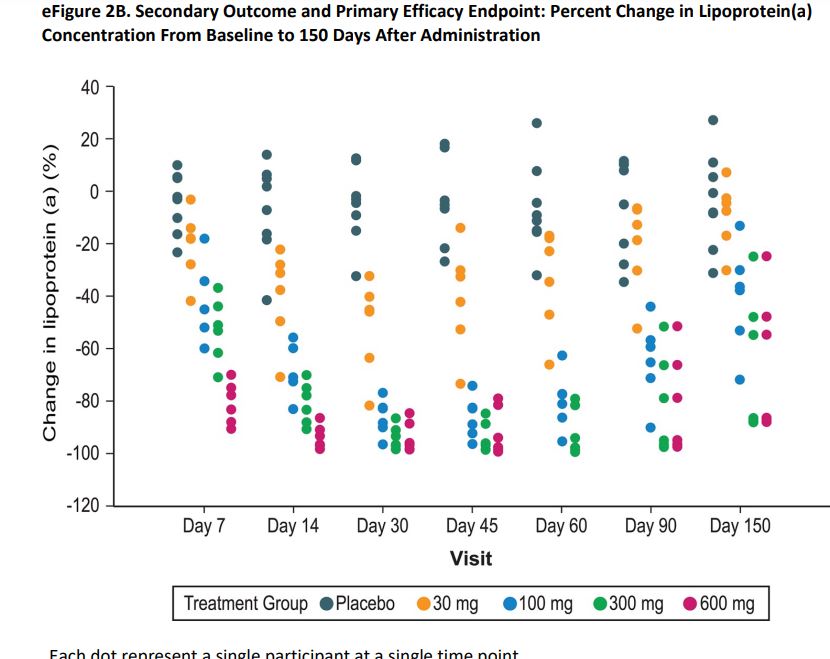

For a more comprehensive understanding: “A siRNA is a double-stranded RNA with guide (antisense) and passenger (sense) strands designed to suppress the translation of a specific target gene. Once the siRNA enters the cell, it is incorporated into an RNA-induced silencing complex. The guide strand and passenger strand are separated, and the passenger strand is cleaved and degraded. The remaining siRNA guide strand binds to the target mRNA, which is degraded by cellular exonucleases, leaving the guide strand intact to degrade additional copies of the target mRNA, resulting in durable effects.”**
I look forward to tracking the clinical trials, maintaining a positive outlook that at least one will reach the market in the coming years.
Nissen provided key education on clinical trials which I feel is beneficial for us all to understand.
“The public needs to know that all clinical trials are monitored by what’s known as a Data Monitoring Committee (DMC). This is a group of usually very senior physician scientists and statistician or two.”
The DMC is “allowed to see the data [as] they are ‘unblinded’ during the trial. They can’t say anything to us about what they know but they can watch what happens,” Nissen said.
“If a trial gets to the point where there is a highly statistically benefit, it is no longer ethical to keep people on placebo and they ‘unblind us.’”
This is the case in any United States clinical trial, for any drug, pertaining to any medical condition. Many of us hear of clinical trials but aren’t familiar with the phases, process, and regulation of them. Stay tuned for a coming article on The Honest Migraine which will provide an overview on clinical trials.
The experts involved, such as Nissen in this Study, at that point would receive a call from the DMC chair that “we voted today that you terminate the trial for overwhelming efficacy.”
Efficacy means the desired result was reached. For all those suffering, for the loved ones supporting them, and for all the experts who expended countless time and energy into developing the drug – it is a victory that can be celebrated by all.
“It means we are going to be able to help patients, which is what we’re here for,” Nissen said.
After that positive milestone and conclusion of the clinical trial, “We have to report in the manuscript, the study report has to go to the FDA, the FDA usually holds an advisory committee meeting, and they get the advice, and they give it a thumbs up or a thumbs down.”
“The FDA usually takes the advisory committee advice and the drug gets approved,” he said.
“It’s a long, maybe more complicated than it needs to be process, but it’s rigorous. And it means we deliver to the public medications that we know are safe and effective.”
The unfortunate event that led to the rigorous process was the Thalidomide crisis, “where a drug was given to women in pregnancy to prevent nausea and a lot of their babies were born without arms or legs,” Nissen said.
“At the time there wasn’t as rigorous standards at the FDA; and one heroic woman kept that drug out of the U.S., but it was used in Europe and a lot of babies were born with limb defects.”
This tragedy “taught the world you have to be careful with drugs, you have to study them carefully, and you have to get good answers (statistically and scientifically) and then it has to go through the rigorous review process,” Nissen explained.
If you don’t know your Lp(a) level – now is a great time to inquire at your doctor’s office. All it takes it a simple check mark on a lab order for a blood draw.
Nissen’s advice? Individuals “have to be aware and keep their eyes on what is going on.”
“If [you] are young and have had a heart attack or a stroke without an explained reason, [you] should almost certainly have Lp(a) checked.”
He continued, “What we do for those people is we treat all their other risk factors really aggressively to try to protect them until we have a therapy on the market.”
I’m grateful for Dr. Nissen shedding more light on this important health matter.
Dr. Steven Nissen is Chief Academic Officer of the Heart, Vascular, and Thoracic Institute at the Cleveland Clinic. He formerly served as President of the American College of Cardiology. Nissen is a recipient of Time Magazine’s World’s 100 Most Influential People. In 2015, he was named by Thompson-Reuters as one of the world’s most highly cited physician-scientists. He is a pioneer in his field.
**Nissen SE, Wolski K, Balog C, Swerdlow DI, Scrimgeour AC, Rambaran C, Wilson RJ, Boyce M, Ray KK, Cho L, Watts GF, Koren M, Turner T, Stroes ES, Melgaard C, Campion GV. Single Ascending Dose Study of a Short Interfering RNA Targeting Lipoprotein(a) Production in Individuals With Elevated Plasma Lipoprotein(a) Levels. JAMA. 2022 May 3;327(17):1679-1687. doi: 10.1001/jama.2022.5050. PMID: 35368052; PMCID: PMC8978050.
Coming next: The power of food: Easy, low cost changes improved my health
